Resources
TIRF microscopy
TIRF microscopy
Total Internal Reflection Fluorescence (TIRF) microscopy is the ideal tool to study cellular processes in the plasma membrane area. Because only a thin layer of the cell above the coverglass is illuminated, the rest of the cell is not disturbed. This fact results in a good signal to noise level, low bleaching, less stress to the cell and enables long time live imaging.
Our new Zeiss TIRF 3 is a laser based microscope that also works in epi-fluorescence mode. The system has 4 high powered lasers for rapid live cell photo bleaching experiments and two sensitive EMCCD cameras with a chromatic splitter for acquiring two fluorescent probes simultaneously. This microscope is equipped with excellent optics for enhanced resolution in TIRF mode and is also fitted with an incubator.
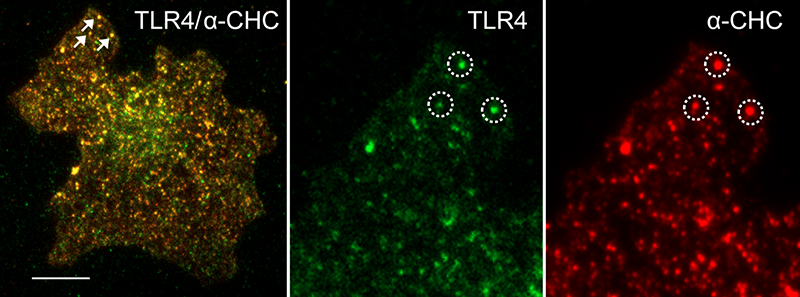 LPS stimulation induces TLR4 punctate structures in the plasma membrane. TIRF images showing co-localization of TLR4 and the clathrin heavy chain 60 min after LPS stimulation in U373-CD14 cells co-expressing TLR4-YFP. Scale bar 5μm.
LPS stimulation induces TLR4 punctate structures in the plasma membrane. TIRF images showing co-localization of TLR4 and the clathrin heavy chain 60 min after LPS stimulation in U373-CD14 cells co-expressing TLR4-YFP. Scale bar 5μm.STED superresolution microscopy
STED superresolution microscopy
STED is the abbreviation of STimulated Emission Depletion and is a technique applied to microscopy, providing better resolution than traditional confocal microscopy. The STED resolution improvements come from a reduction of the physical dimensions in the focal spot from where the fluorescence can be emitted. This is achieved by introducing a depletion donut mask overlapping the diffraction-limited spot in the focus plane. By increasing the laser power of the depletion laser the donut gets bigger and consequently the spot gets smaller. This is achieved in all three dimensions.
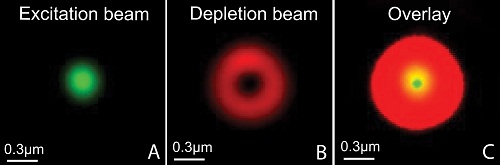 The STED principle. The resolution improvements is achieved by introducing a so-called depletion-“donut” mask overlapping the normal (diffraction-limited) spot in the focus plane. By increasing the laser power of the depletion laser the “donut” gets bigger, and consequently the spot gets smaller.
The STED principle. The resolution improvements is achieved by introducing a so-called depletion-“donut” mask overlapping the normal (diffraction-limited) spot in the focus plane. By increasing the laser power of the depletion laser the “donut” gets bigger, and consequently the spot gets smaller. Our new Leica SP8 STED 3X microscope is equipped with a pulsed white-light laser that works in conjunction with an AOBS (acoustic optical beam splitter) providing any wavelengths between 470 and 670nm. The STED is provided by 3 powerful depletion lasers with wavelength 592, 660 and 775nm covering fluorophores of most of the visual spectra. It is fitted with sensitive hybrid detectors (HyDs) with time gating of the fluorescence signals and a 3D vortex for adjusting the ratio between the lateral and axial resolution. The microscope also has a high speed resonant scanner, a fast piezo z-drive and an incubator for live cell imaging.
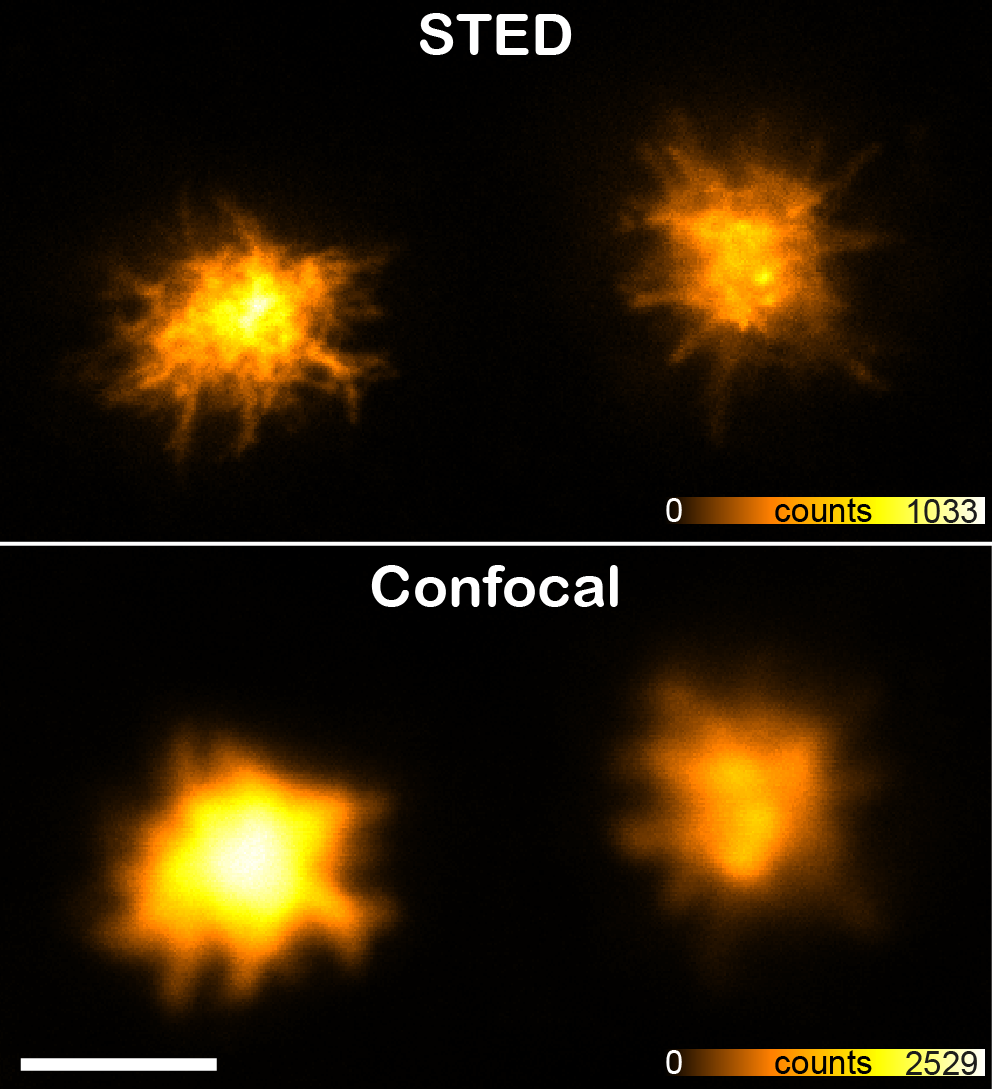 top: confocal, bottom: STED. Immortalized murine macrophages expressing mCherry-ASC were stimulated with 10 µg/ml Nigericin in order to induce formation of ASC speck inflammasomes in the cytoplasm. These structures have prionoid activities and are responsible for the maturation of the cytokine interleukin 1β. Scale bar 2μm
top: confocal, bottom: STED. Immortalized murine macrophages expressing mCherry-ASC were stimulated with 10 µg/ml Nigericin in order to induce formation of ASC speck inflammasomes in the cytoplasm. These structures have prionoid activities and are responsible for the maturation of the cytokine interleukin 1β. Scale bar 2μmContact and Service Request
Contact and Service Request

Manager
Bjørnar Sporsheim
Phone: +47 93033045
Email: bjornar.sporsheim@ntnu.no
CMIC-ALM location
Kunnskapssenteret, 3rd Floor (3. etg.)
St. Olavs Hospital
Olav Kyrres gate 10
7030 Trondheim
Norway
CMIC-Histology location
Laboratoriesenteret, 4th Floor East (4. etg)
St. Olavs Hospital
Erling Skjalgsons gate 1
7030 Trondheim
Norway
Delivery address
NTNU/IKOM
Logistikksenteret Helse Midt Norge
Industriveien 59
Internt: Gastrosenteret 3.etg. sør
7080 Heimdal

