Loyford Muchui
Loyford Muchui
Loyford Muchui
Title of the project: Fundamental Mechanisms of C(-A)-S-H Carbonation
Project description
Background
According to Global Cement and Concrete Association, cement is the world’s most produced material with a production estimate of 4 billion tonnes yearly, leading to an annual CO2 emission of over 1.6 billion tonnes (GCCA, 2021). This makes cement production one of the major contributors to CO2 emissions, responsible for about 8% of the global anthropogenic CO2 emissions (Fateh Belaid, 2022). Carbon Capture and Utilization (CCU) is one of the most effective methods of mitigating the negative environmental impacts attributed to the cement production. Calcium Carbonate Cements (CCCs) produced through carbonation curing and cement recarbonation, was described by Zajac et al. (2022) as one of the most effective strategies for CCU. To aid in the utilization of CO2 in calcium carbonate cements, the CONTRABASS project focuses overall on the fundamental physico-chemical processes governing the carbonation of the clinker phases and the cement paste, and the subsequent formation of calcium carbonate cements.
In CONTRABASS, carbonation curing, cement recarbonation and calcium carbonate engineering are studied. Our project is part of WP2 on cement recarbonation, so studying the reabsorption of CO2 by cement. Figure 1 shows an illustration of the CCU through carbonation curing and cement recarbonation, highlighting the overall focus of the CONTRABASS project and the focus of this PhD project.
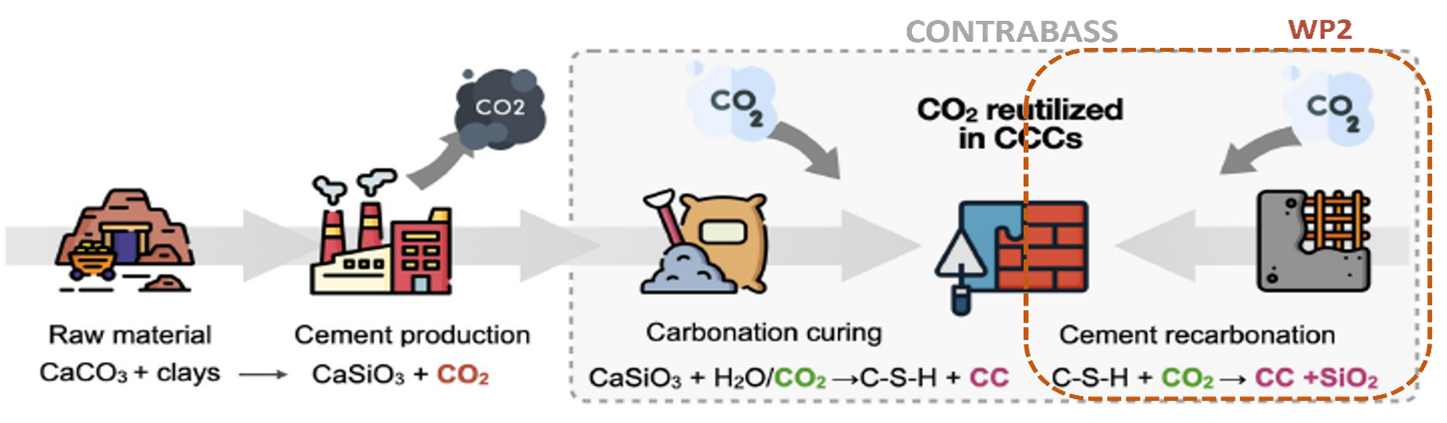
Figure 1. Carbonation curing and cement recarbonation as a method of CCU (Contrabass, 2023)
C(-A)-S-H makes up around 60-70% of the final hydrated product in cement (Tang et al., 2021). It is therefore important to study the mechanism of carbonation of this main cement hydration product, and its contribution in cement recarbonation. Several studies have been conducted on carbonation of C(-A)-S-H (Sevelsted & Skibsted, 2015; Lu et al., 2023) . However, from a fundamental point of view, several aspects of the C(-A)-S-H carbonation process are not yet well understood, which limits faster advances. For instance, there is limited knowledge on which is the limiting factor for the carbonation reaction, between dissolution of C(-A)-S-H and precipitation of the carbonate products. Understanding the limiting factor in the carbonation process, will help in the tailoring of CCCs to ensure sufficient reactivity and performance.
General objective
The primary aim of the project is to understand the fundamental mechanisms of C(-A)-S-H carbonation under saturated conditions.
Specific objectives
- To synthesize C(-A)-S-H with different Ca/Si and Al/Si ratios.
- Develop both a closed and a flow-through carbonation reactor to study the carbonation mechanism of C(-A)-S-H.
- Study the reaction kinetics of the carbonation reaction and determine whether the overall carbonation rate is limited by the leaching of Ca2+ from C-S-H or by precipitation of the carbonate phases.
Research questions
- How is the carbonation of CASH affected by the type of carbonation reactor; comparison of a closed and a flow-through reactor.
- What is the effect of Ca/Si ratio on C(-A)-S-H carbonation?
- What is the carbonation limiting factor between dissolution and precipitation of Ca2+ and CO32- ions?
- How does the carbonation behavior of pure C(-A)-S-H correspond with that of hydrated cement paste in either closed or flow-through reactors?
Deliverables
- Propose a protocol for C(-A)-S-H synthesis
- Develop a protocol for closed carbonation reactor
- Develop a protocol for flow through carbonation reactor
- Report on the first set of experimental results for both solution and solid characterization.
Methodology
First, the dissolution of synthesized C(-A)-S-H will be studied in a closed carbonation reactor with a high liquid to solid ratio, as illustrated in Figure 2. The saturation indices will be monitored in the system to ensure that we don’t have precipitation. The conditions will then be altered to allow for precipitation. In both cases, the exposure solutions will be continuously monitored (e.g. conductivity, pH, Ca2+ concentration). In addition, the solution and solids will be sampled for analysis as the carbonation process progresses.
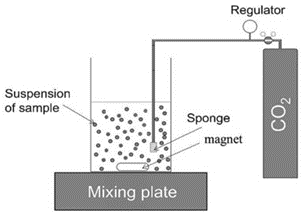
Figure 2: An illustration of the closed C(-A)-S-H carbonation set-up (Lu et al., 2023)
Secondly, a selection of C(-A)-S-H composition will also be tested in a flow through cell reactor, to study the dissolution in an exposure solution with a constant composition.
Finally, a selection of hydrated cement paste suspensions will be investigated using both or either of the two carbonation reactors, to elucidate the carbonation mechanism in cement paste.
Research Plan and Collaboration
| Secondment host | Aim of the secondment | Tentative period |
| Oulu University, Finland | To carry out solid analysis of the synthesized C(-A)-S-H by solid-state NMR | Spring 2025 |
| Centre National De La Recherche Scientifique (CNRS1), France |
To perform potentiometric titration and solubility experiments of C(-A)-S-H and alumino-silicate gels. Knowledge transfer on monitoring set-up of carbonation reactor |
Autumn 2025 |
| Heidelberg Materials, Germany | Industrial experience on Carbonation reactor setup and experimental protocol | Spring 2026 |
Funding: The PhD project is a part of the MSCA doctorate network project called CONTRABASS (www.contrabass-msca.eu), funded by the European Union’s Horizon Europe research and innovation programme.
Date of start of PhD: June 2024
Internal supervisors:
External supervisors:
- Maciej Zajac - Heidelberg materials, Germany
- Christophe Labbez - Centre National De La Recherche Scientifique (CNRS), France
