Advanced Optoelectronic Nanomaterials
Advanced Optoelectronic Nanomaterials
The group focuses its research on the synthesis of functional molecules for incorporation into optoelectronic devices.
The main focus is on the design and synthesis of the materials, as well as the evaluation of their performance in actual devices.
The materials of interest are divided into two main categories
- Semiconducting Organic Molecules and Polymers
- Carbon nanostructures
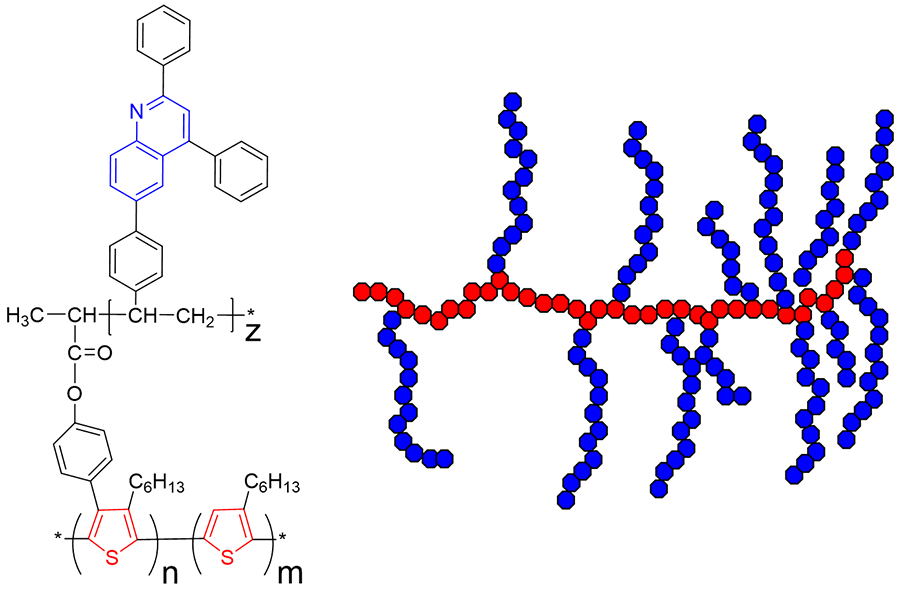
Semiconducting Polymers
Based on the discovery in the 70’s by Alan Heeger, Alan MacDiarmid and Hideki Shirakawa (Nobel Prize in Chemistry, 2000) that doped polyacetylene can conduct electricity, a new class of materials was introduced.
Semiconducting polymers can be used in a variety of applications ranging from nanomedicine to optoelectronics. Known also as “conductive plastics” these materials pioneered a new era for electronics as they harmoniously couple the properties of inorganic semiconductors with the durability, flexibility, lower cost and processing advantages of polymers.
Due to the fact that chemical tailoring allows for the incorporation of a wide array of organic moieties (e.g. thiophene, carbazole, fluorene, quinoline, pyrrole, furan etc) the final properties of the polymer can be fine-tuned providing materials with, virtually, infinite potential.
For semiconducting organic molecules and polymers, the true advantage lies in their scalability and processability. Materials no longer need to be sourced out and mined from the earth, but rather synthesized in the lab using established organic synthetic techniques. As for the devices, the higher cost of e.g. Silicon device fabrication requiring ultra clean lab conditions and higher temperatures is virtually negated by the organic material’s solution processability, allowing for a variety of deposition techniques such as spin coating, gravure printing, ink jet printing etc.
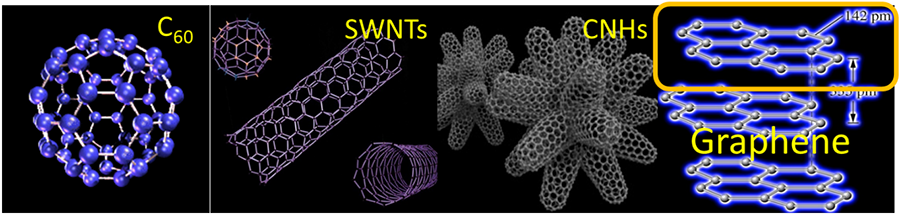
Carbon nanostructures
Until the 80s allotropic forms of carbon included graphite, diamond and of course, the amorphous coal. However, the discovery of a round “nano-football” comprised of 60 carbon atoms in 1985 by Robert Curl, Jr, Harold Kroto and Richard Smalley (Nobel Prize in Chemistry, 1996) ushered in a new era for these nanomaterials. The structure was named buckminsterfullerene as a tribute to architect Buckminster Fuller who had used a similar structure at his 1967 Montreal World Exhibition building. Similar truncated spheres of carbon comprised of 70, 84 atoms were also reported later on and this material proved to possess remarkable properties. Chemical modification on C60 fullerene allowed for soluble materials, as well as interesting modifications of the original, in the form of azafullerene, (C59N, where one carbon atom of the structure is replaced by a nitrogen) or a host of endohedral fullerenes (where several metal atoms can be “trapped” inside the carbon cage). Each of these materials possess different properties and opens up pathways to different applications.
In the 90’s two more carbon allotropes were discovered by Sumio Iijima in the form of carbon nanotubes and carbon nanohorns. These tubular forms basically retain all the interesting electronic properties of fullerenes but possessed, also, remarkable mechanical properties. Carbon nanotubes (CNTs) are synthesized as individual tubes, while carbon nanohorns (CNHs) are formed as uniform spherical bundles (~100nm diameter) of carbon nanotubes with conical edges that resemble a dahlia-like structure.
In 2004, Andre Geim and Konstantin Novoselov (Nobel Prize in Physics 2010) isolated a single 2D layer of carbon atoms from graphite, which was up till then was theorized “could not exist” due to instability. The introduction of graphene sparked renewed interest for chemists and physics and opened new potential applications based on the newest member in the family of carbon allotropes.
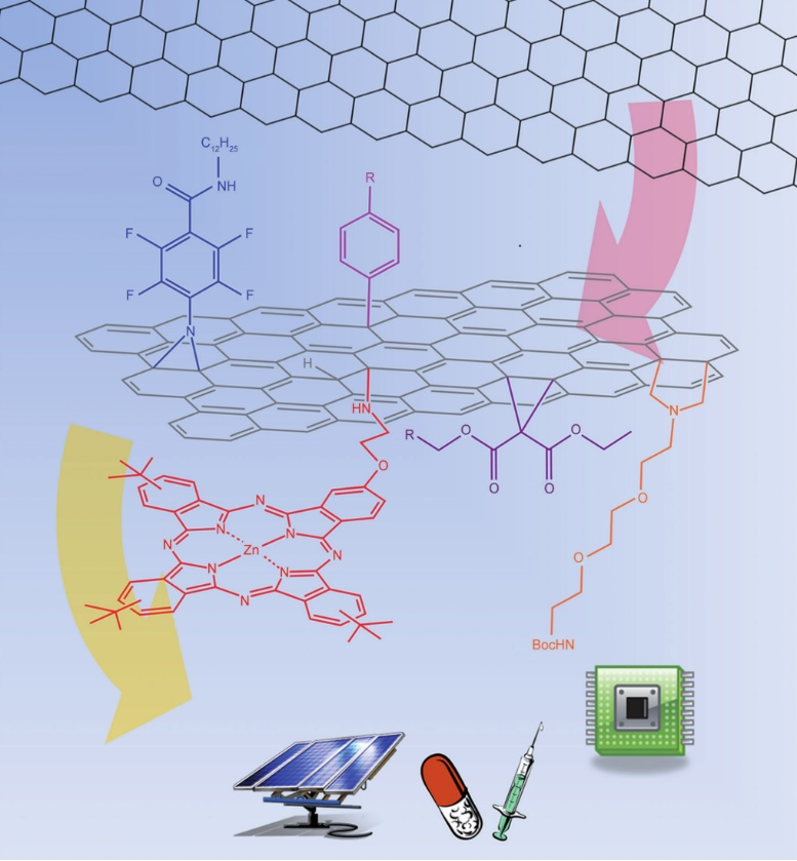
Decoration of carbon allotropes through classical organic chemistry with various molecules can yield hybrid materials that possess the properties of both the organic addend and the carbon nanostructure and in some cases, even introduce new properties to the resulting product.
* Economopoulos, et al. Macromolecules 2007, 40 (4), 921-927
** Economopoulos, S. P.; Tagmatarchis, N., Chem.–Eur. J. 2013, 19 (39), 12930-12936.
